noh lewis structure
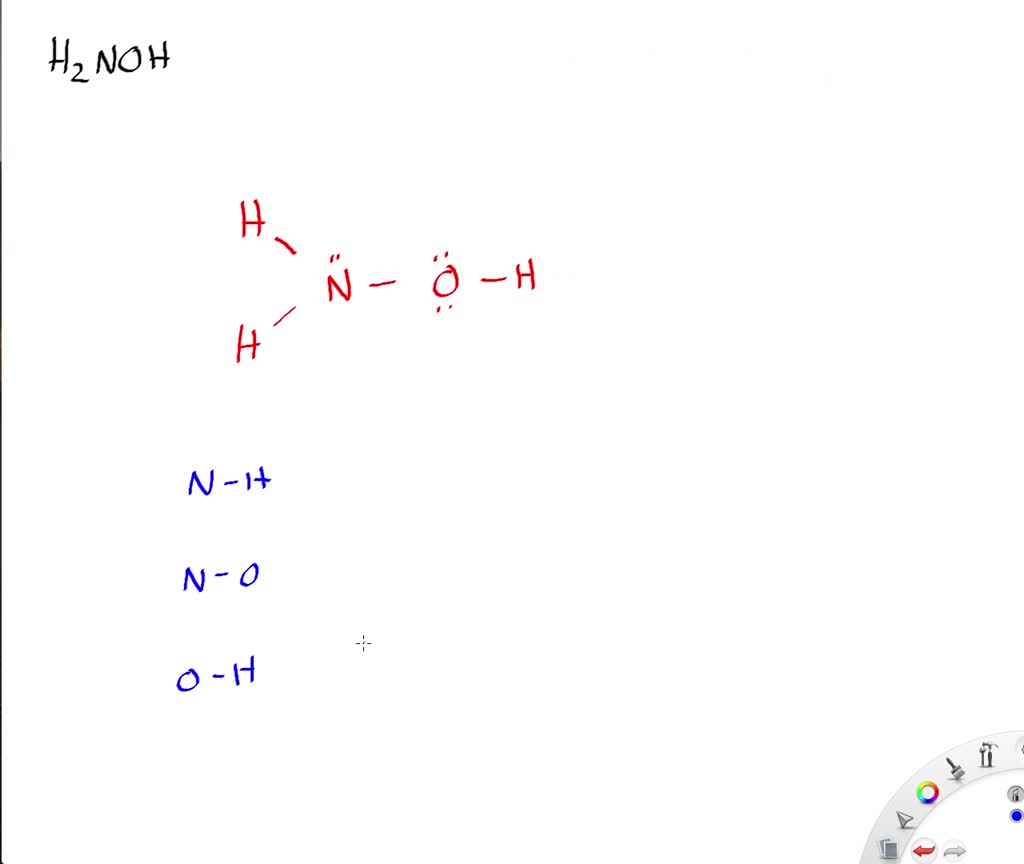
Draw the lewis structure for Livermorium diiodide. Draw the Lewis structure for hydroxylamine H 2 NOH.

Nh2oh Lewis Structure How To Draw The Lewis Structure For Nh2oh Hydroxylamine Youtube
Check the stability and minimize charges on atoms by.
. The nitrogen is the central atom and there is one lone pair on it. Draw the lewis structure for Moscovium pentabromide. The trial structure has three extra electrons.
Follow some steps for drawing the lewis dot structure for NH2OH. Put the least electronegative atom in. Step 1 of 5.
Lewis structure of NO. The NO2 Lewis structure has a total of 17 valence electrons. A step-by-step explanation of how to draw the HNO Lewis Dot StructureFor the HNO structure use the periodic table to find the total number of valence electr.
Total electrons pairs as lone pairs and bonds. What orbitals overlap to form the bond between nitrogen and oxygen. Noh can be divided into five different categories.
Count total valence electron in NH2OH. Draw the lewis structure for OganessonOGdifluoride. In order to find the total valence electrons in a NOCl molecule first of all you should know the valence electrons present in nitrogen atom.
Your source for diversity of noh lewis structure articles. In between each noh a separate kyōgen play would be performed. Determine the total amount of valence electrons.
Do you know that the elements. Lewis structures are diagrams that represent the chemical of covalently bonded molecules and coordination compounds. In the Lewis structure for NOF there are a total of 18 valence electrons.
Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule. Lewis structures were first introduced in 1916 by Gilbert Lewis and have been adopted as standard fare in high school and college chemistry courses ever since. This will mean that it.
Its not common to have an odd number of valence electrons in a Lewis structure. Most stable lewis structure of NO is shown below. The lewis structure of NH2OH has a total of 3 lone pairs and 4 bond pairs.
Draw the Lewis structure for hydroxylamine H 2 NOH. In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. A Lewis structure also called Lewis dot formulas Lewis dot structures or electron dot structures are pictorial diagrams that represent the bonding between atoms in a compound and the placement of electrons.
Mark charges if there are. Lets go through the rules for making Lewis structure using nitrogen dioxide as our test example. Minimize charges again if there are Lets break down each step in detail.
Lewis Structure of NO2. 9 - Draw the Lewis structure for hydroxylamine H2NOH. The NOF Lewis structure is very similar to NOCl and NOBr.
Steps of VSEPR rule to draw lewis structure of NO. 9 - Draw the Lewis structure for 1. To draw the Lewis structure first draw the bond skeleton.
What is the hybridization for nitrogen and oxygen in this molecule. Inside an atom we have the positively charged nucleus surrounded by electrons in their shells forming a. Also there is an unpaired electron on nitrogen atom.
Separate added sketchers with signs from the dropdown Question. The nitrogen exhibits a tetrahedral geometry because to the sp3 hybridization. Steps of drawing NOCl lewis structure Step 1.
For HNO 3 molecule its lewis structure and those steps are explained in detail in this tutorial. O2SOH2 OPOH3 SF3CF3 NH2OH Deduce the Lewis structure for the species shown based on their formula. With three electrons we can make only one double bond with one electron left over.
Find the total valence electrons for the NO molecule. ----- Steps to Write Lewis Structure for compounds like NO ------ 1. This is known as goban date.
Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Cyanamide appears as colorless deliquescent crystals. Due to lone pair electron compression the H-N-H and H-N-C bonds angles are shorter than the standard 1095o.
Heres how you can draw the NO lewis structure step by step. Following VSEPR rule steps are followed to draw the lewis structure of NO and they are explained in detail in. The valence electrons you have available are.
The first step is to figure out how many electrons you diagram should have. There is a double bond between nitrogen and oxygen atom. Draw one structure per sketcher box.
Find the total valence electrons in NOCl molecule. The C-N sigma bond is formed when one of the sp3 hybridised orbitals collides with an sp3 hybridised orbital from carbon. God man woman mad-woman demon.
Find total number of electrons of the valance shells of hydrogen nitrogen and oxygen atoms. Draw Lewis structures for the N-methylidenehydroxylamine molecule CH2NOH the methylamine molecule CH3NH2 and the acetonitrile molecule CH3CN in the window below and then answer the questions that follow based on your drawings. The tradition of gobandate was developed in the Edo period.
Check the formal charges to be sure that each atom has a formal charge of zero. We have to insert one or more double bonds. Because of this well try to get as close to an octet as we can on the central Nitrogen N atom.
NH2OH lewis structure has two N-H bonds one N-O bond and one O-H bond. The trial structure is You have 14 valence electrons in your trial structure. 1 N 1 O 15 16 11.
Mark lone pairs on atoms. In a full noh program on noh from each category would be played. From the electron arrangement around the central atom identify all species with unhybridized central atom hybridization.
A Lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Lewis Structure A Brief Intro.

Nh2oh Lewis Structure Hydroxylamine
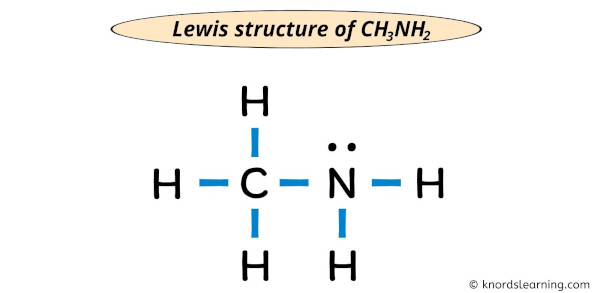
Lewis Structure Of Ch3nh2 With 6 Simple Steps To Draw
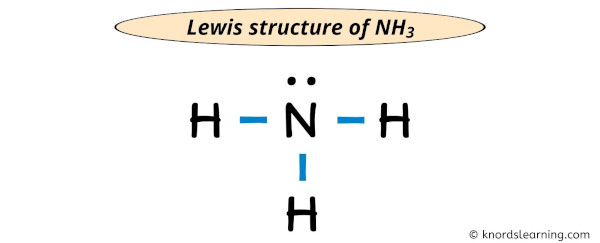
Lewis Structure Of Nh3 Ammonia With 6 Simple Steps

Lewis Dot Structures How To Calculate The Number Of Lone Pairs Using A Formula Youtube
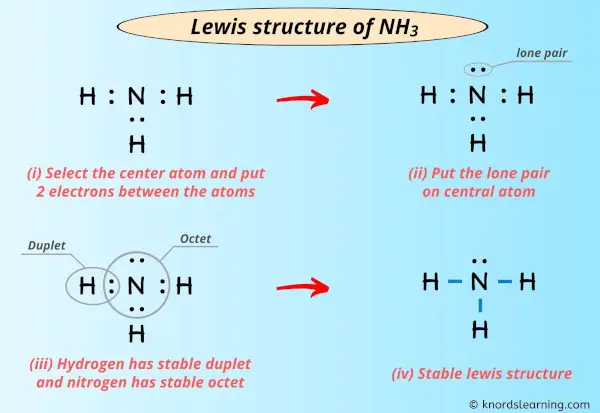
Lewis Structure Of Nh3 Ammonia With 6 Simple Steps

Hcn Lewis Structure Hydrogen Cyanide
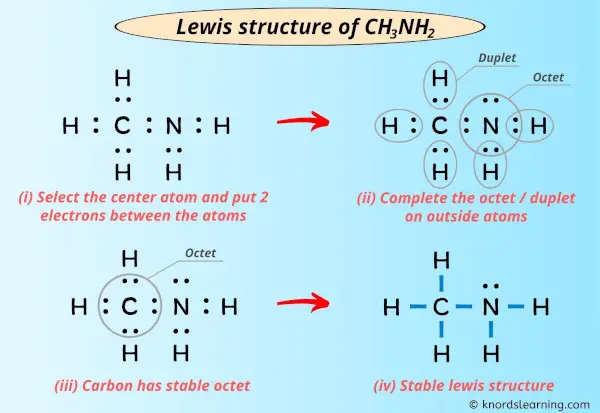
Lewis Structure Of Ch3nh2 With 6 Simple Steps To Draw
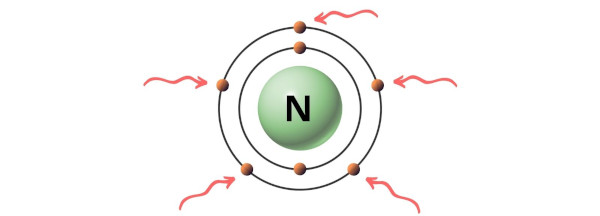
Lewis Structure Of Nh3 Ammonia With 6 Simple Steps

Nh2oh Lewis Structure How To Draw The Lewis Structure For Nh2oh Hydroxylamine Youtube
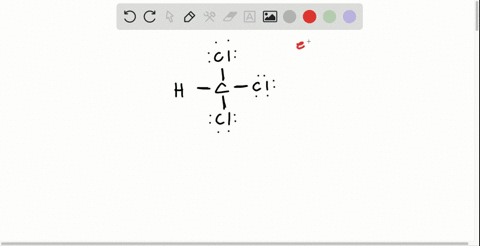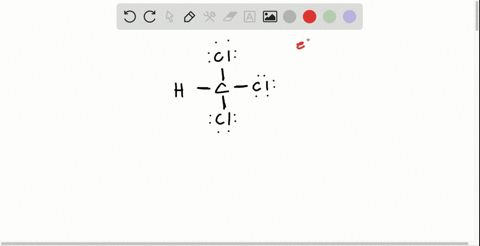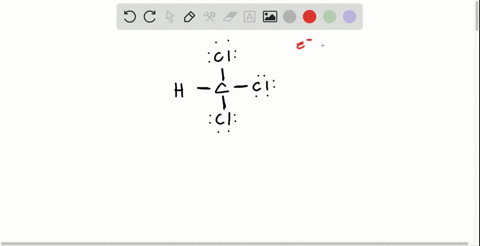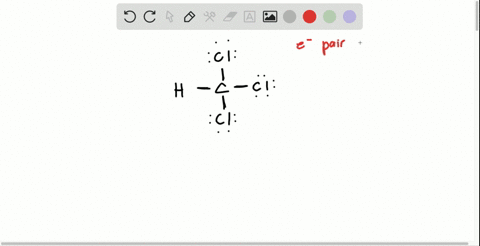
Solved Draw The Lewis Structure For Hydroxylamine Mathrm H 2 Mathrm Noh What Is The Hybridization For Nitrogen And Oxygen In This Molecule What Orbitals Overlap To Form The Bond Between Nitrogen And Oxygen

Ch2o Lewis Structure Methanal Or Formaldehyde Molecules Methanal Lewis

Solved Write A Lewis Structure Of The Hydroxylamine Molecule Mathrm H 2 Noh Then With Data From Table 10 2 Determine All The Bond Lengths

Of2 Lewis Structure Oxygen Difluoride
Why Does The Lewis Structure Of Hno3 And Have A Positive Charge Quora

Xeo3 Lewis Structure Xenon Trioxide
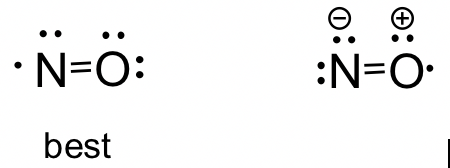
1 2 Lewis Structure Organic Chemistry I


0 Response to "noh lewis structure"
Post a Comment